Members from multiple therapeutic areas will incorporate the patient voice into clinical trial technology design.
Newsroom
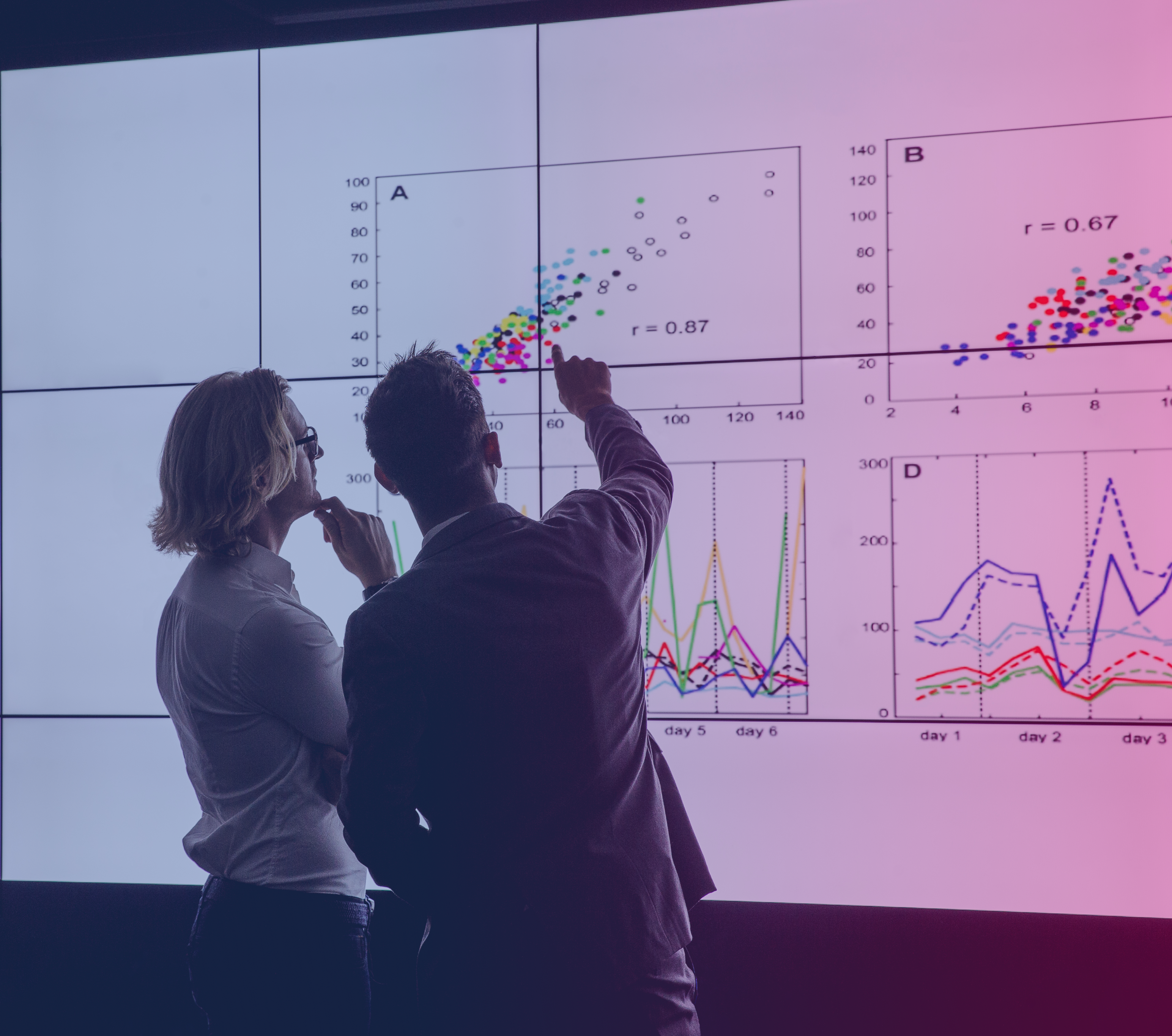
AI tool provides real time eCOA and connected device operational data insights.
Industry-leading behavioral science tool SPUR™ is validated to predict adherence and will be offered through the Clinical ink technology platform
Clinical ink, a global life science technology company, announces the promotion of John Pappadakis from EVP, Global Business Development to Chief Commercial Officer and Megan Petrylak from EVP, Clinical Operations to Chief Operating Officer.
Clinical ink announces EDCXtra: An integrated web-based EDC, DDC and eCOA platform.
Clinical ink announced the appointment of Dr. Nicholas Alp as Chief Medical Officer will lead the creation of innovative solutions and commercial strategies for clinical trial technology based on his experience as an interventional cardiologist and clinical trialist.
Clinical ink announces an integrated suite of eClinical tools for cardiometabolic clinical trials.
Clinical ink is expanding its patient engagement suite with the inclusion of the SPUR™ behavioral diagnostic tool created by Observia.