FACT SHEET
Gain a full range of validated eCOA and ePRO questionnaires across a wide range of therapeutic areas for all phases of clinical trials.
Traditional electronic clinical outcome assessment (eCOA) platforms and applications often underperform.
Too complex and time-consuming, data is inaccurately captured, sites are encumbered with inefficient workflows, and dissatisfied patients drop out of studies before eCOAs get completed.
Get your study-specific cost comparison between paper COA and eCOA within 48 hours. See how much you can save today.
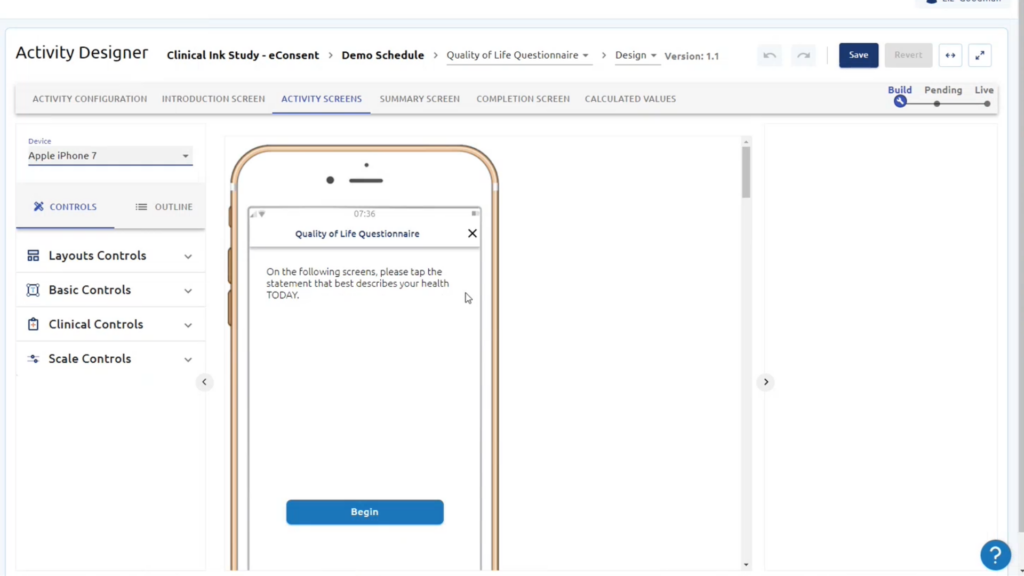
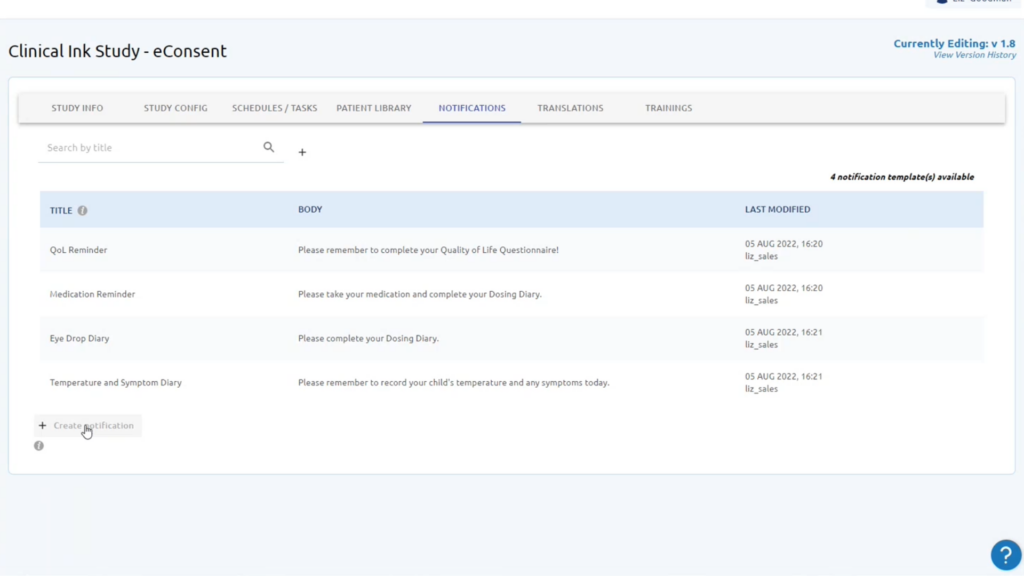
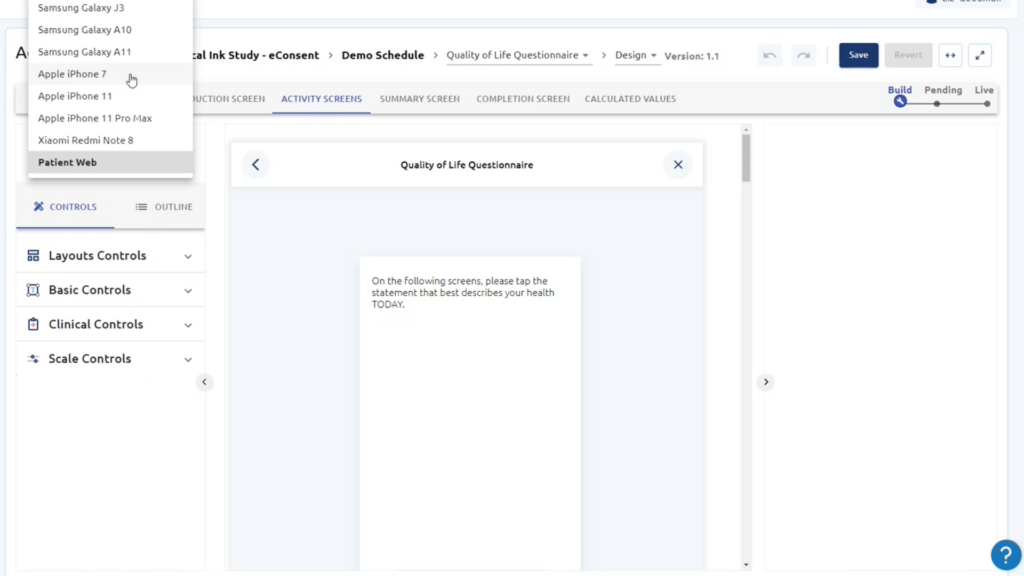
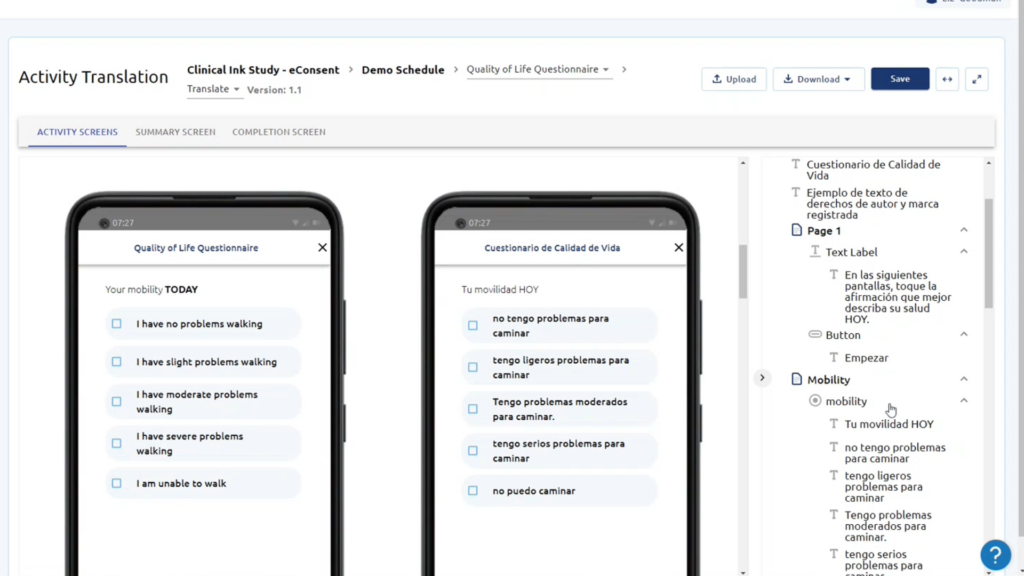
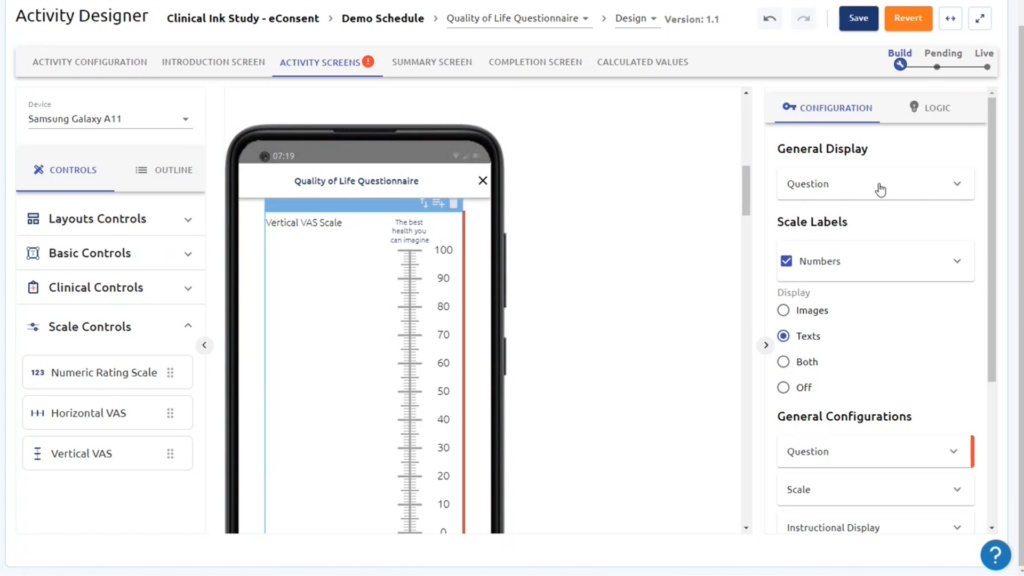
Gain a full range of validated eCOA and ePRO questionnaires across a wide range of therapeutic areas for all phases of clinical trials.
eCOA (electronic Clinical Outcome Assessment) refers to the use of electronic devices, such as smartphones or tablets, to collect patient-reported data in clinical trials. eCOA improves clinical trials by enhancing data accuracy, patient compliance, and overall trial efficiency.
Using eCOA in clinical research offers several advantages. It enables real-time data capture, reduces transcription errors, improves patient engagement, enhances data quality, and allows for remote monitoring of patient outcomes. These benefits lead to more reliable and efficient clinical trials.
eCOA technology allows for the collection of a wide range of patient-reported data, including symptoms, quality of life assessments, adherence to treatment, pain intensity, patient diaries, and more. It provides researchers with valuable insights into patient experiences and treatment outcomes.
eCOA offers advantages over paper-based assessments, making it a preferred choice for clinical trials. Benefits include:
Yes. By supporting Bring Your Own Device (BYOD), Clinical ink offers flexibility and convenience. Patients can use their familiar devices, eliminating the need for additional hardware provisioning. Clinical ink ensures data security and privacy by implementing robust encryption and authentication measures, safeguarding patient information throughout the BYOD process.
Yes, Clinical ink supports the integration of connected sensors and devices for real-time data collection in clinical trials. This includes wearable in clinical trials biosensors, activity trackers, and other medical devices. The integration enables objective measurements, monitoring vital signs, and provides valuable insights into patient health and treatment outcomes. Clinical ink ensures data security and compliance throughout the process, enhancing the depth and accuracy of collected data for more meaningful clinical trial results.
Sales and Solutions
Direct 1.336.464.0697
Support
Toll-Free 1.800.301.5033
Direct 1.336.464.0697
Insert HTML text here.
One Solution, Onsite or Remote
Gain a full range of validated eCOA and ePRO questionnaires across a wide range of therapeutic areas for all phases of clinical trials.
Request more information, submit questions, set up a demonstration, and more.