Dr. Djurhuus commented, “I am excited to join Clinical ink at a time when the convergence of digital health, clinical science, and patient‑centric data capture is creating new opportunities. I look forward to helping advance a disease management solution that delivers meaningful impact for patients while strengthening evidence generation for biopharma partners.”

GlucoseReady™: Diabetes
GlucoseReady™:
Weight Management
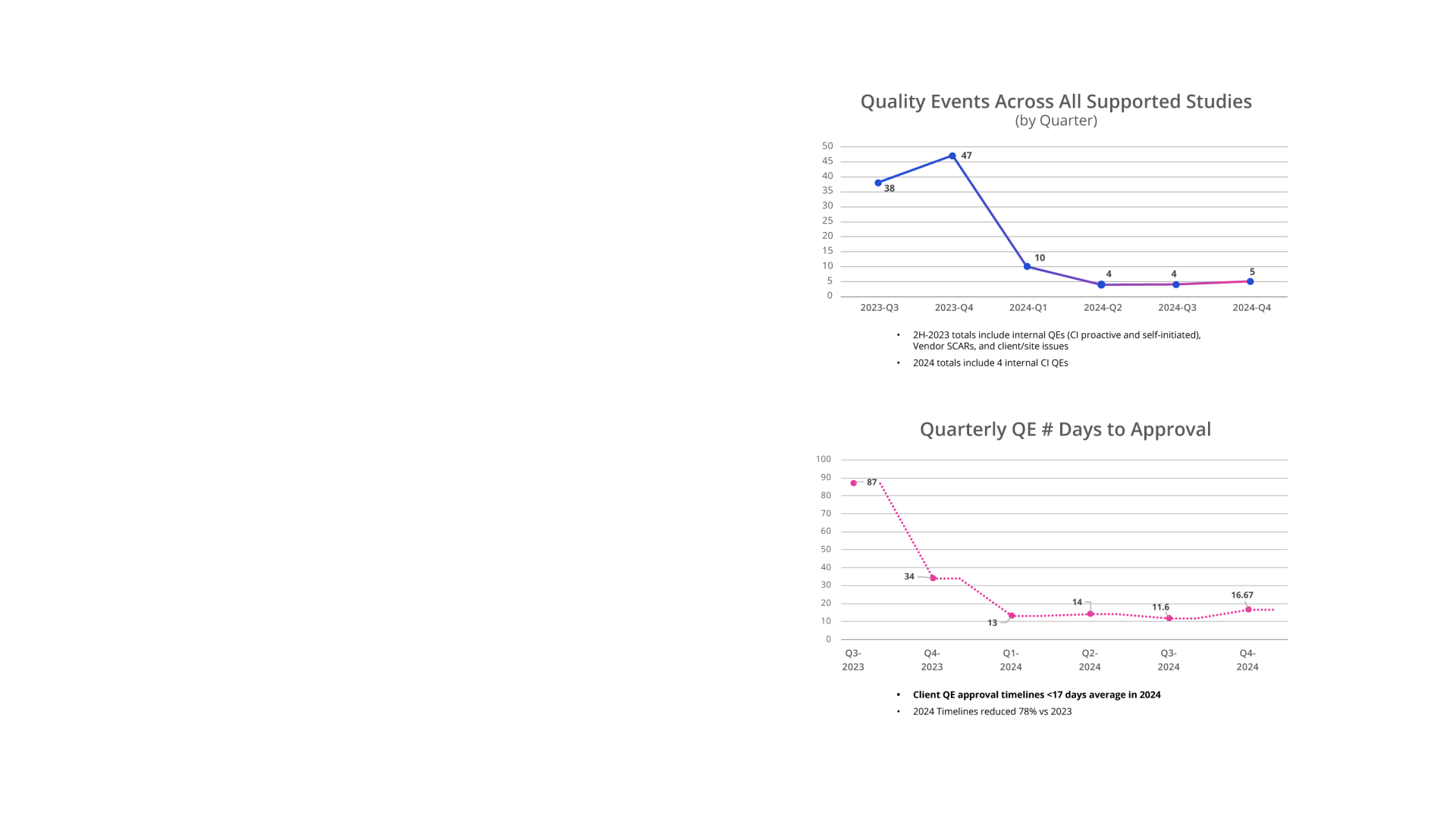
• Commissioned proactive 3rd-party SDLC audit vs evolving EMA / FDA Regulations in 2023 and 2024: 0 Critical findings.
• Master control eQMS system implemented for QMS, LMS, vendor management, quality investigation management.
• All employees maintain 100% monthly LMS compliance.

WEBINAR ON DEMAND
How Patient-Centered Technologies Can Elevate Diabetes Clinical Trials to Align with Current Standards of Care

Reduce your Study Build Timeline to 10 Days

Nature Magazine Publishes Work Demonstrating Clinical ink Technology Sensitivity to Parkinson’s Disease

COA vs. eCOA Cost Calculator

Breakthrough Evidence with Digital Biomarkers in Movement Disorders
Clinical ink is leading the way to recenter decentralized clinical trials by connecting data and operations.
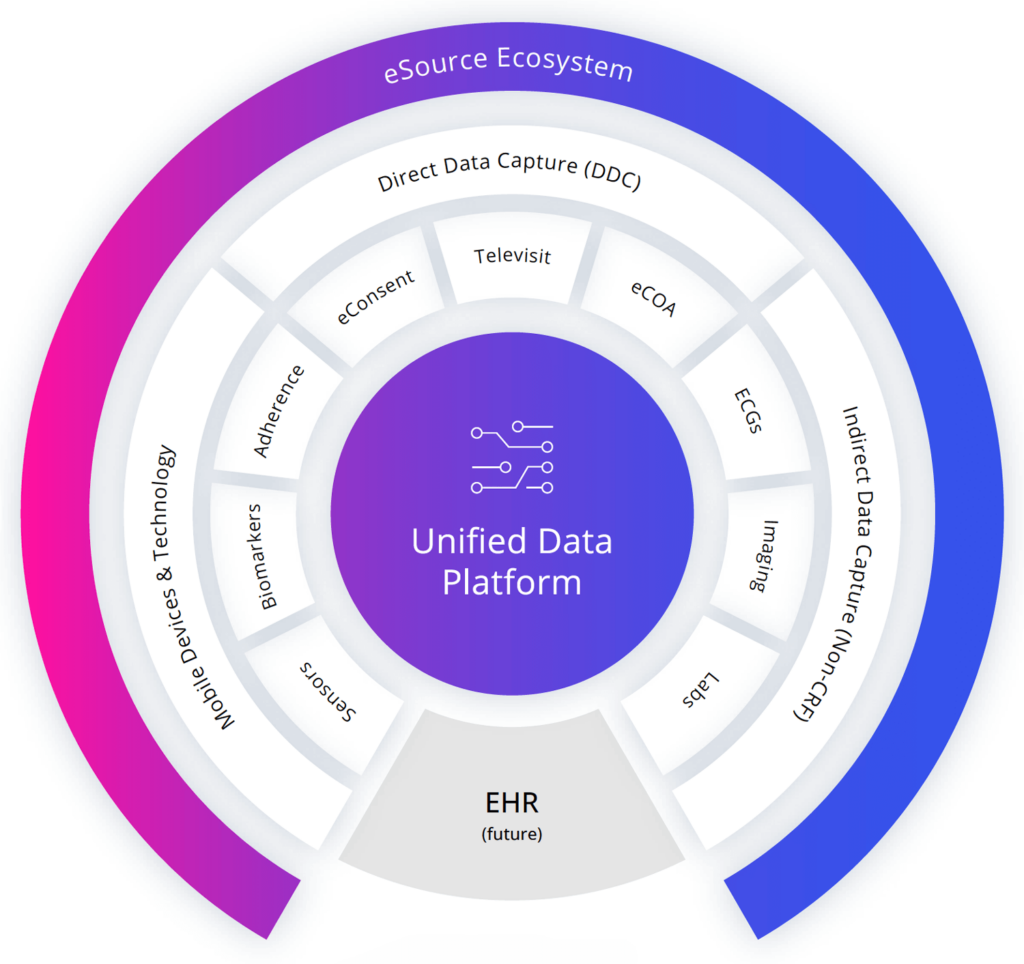
At the core of our clinical trial solutions is the Clinical ink eSource ecosystem, designed to ingest all incoming data into a unified data platform that supports analytics within and across studies, regardless of how it was captured or by whom.
A Better eSource Solution
Clinical ink’s eSource methods integrate Direct Data Capture (DDC) technology and electronic Clinical Outcome Assessments (eCOA) for a wide range of phases and therapeutic areas.
The result is faster, higher-quality clinical trial data. Our eSource solutions ingest data from multiple sources, facilitating decentralized trials and shifting the industry towards capturing data directly from the patient.
Lorem Ipsum sit Amet Dolor
Clinical ink’s eSource methods integrate Direct Data Capture (DDC) technology and electronic Clinical Outcome Assessments (eCOA) for a wide range of phases and therapeutic areas.
The result is faster, higher-quality clinical trial data. Our eSource solutions ingest data from multiple sources, facilitating decentralized trials and shifting the industry towards capturing data directly from the patient.

and Instruments
eSource Technology
eCOA
DDC Technology
eConsent
Simplify and speed study startup, standardize processes, and empower patients.
eLAS® Suite
Facilitate lupus trials with straightforward, efficient, and reliable direct data capture.
Mobile Devices
Televisit
Benefit from an easy, efficient, holistic telehealth solution and improve remote monitoring.
Sensors & Wearables
Enrich advanced clinical trial data collection with a variety of sensors and wearables solutions.
Digital Biomarkers
Validate measurements from sensors, algorithms, and deliver clinical data in real-time.
See everything our eSource technology platform has to offer.
eSource technologies improve the efficiency and reduce the cost of clinical trials, particularly in the context of complex therapeutic areas.
The depth and breadth of our therapeutic experience – across neurology, infectious disease, immunology, and oncology – means you get the expertise and improved results for your clinical research.
Neurology
Improve results for complex clinical trials involving central nervous system conditions.
Infectious Disease
Speed up trial timelines and improve the data quality of your infectious disease studies.
Immunology
Leverage eSource tools to simplify and improve outcomes in complex Lupus clinical trials.
Oncology
Deliver on the Enhancing Oncology Model and improve patient adherence throughout the trial.
Diabetes
Immunology
Leverage eSource tools to simplify and improve outcomes in complex Lupus clinical trials.
Infectious Disease
Speed up trial timelines and improve the data quality of your infectious disease studies.
Neurology
Improve results for complex clinical trials involving central nervous system conditions.
Neurology
Improve results for complex clinical trials involving central nervous system conditions.
Oncology
Deliver on the Enhancing Oncology Model and improve patient adherence throughout the trial.
It is amazing how you have been able to assist remotely.
Your careful analysis, step-by-step problem-solving, and willingness to consider varied options are much appreciated.
eCOA, Phase II.b
Your eLAS® suite has become his gold standard, and the rapid modifications made to the original version impressed him quite a bit.
We have not found another provider like you and continue to recommend Clinical ink as a preferred partner.
eCOA + ePRO
20+ studies completed with Clinical ink
A compliment from the pharma team: They are very happy with your responsiveness.
They realize that the closeout process has been very intensive and there’s been a lot of back and forth. They mentioned Clinical ink is working very diligently to get everything resolved.
Senior PM, Cognitive Assessment Company
eCOA, Phase II and III
Responsiveness, flexibility, and subject matter expertise. These have been nothing less than impressive during this time of chaos and uncertainty.
Thank you for your leadership, and the example your organization sets for the industry.
VP, Innovation
Pharma
40+ studies completed with Clinical ink



