FACT SHEET
Introducing GlucoseReady™: Transforming Cardiometabolic Trials
Download our fact sheet to learn more.
Experience the future of diabetes, obesity and NASH/MASH research with our integrated digital platform.
Real-Time Data Capture
Meeting new FDA guidelines for seamless and immediate insights
Comprehensive eCOA Integration
From patient onboarding to in-clinic visits and reporting
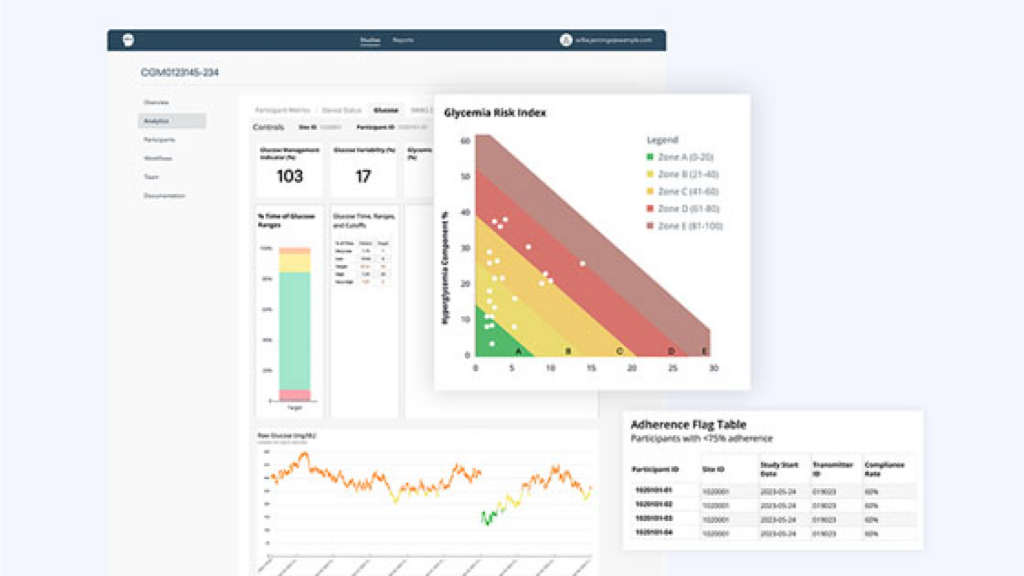
Advanced Biomarker Analysis
Powered by Continuous
Glucose Monitoring (CGM) and Blood Glucose Meters (BGM)
GlucoseReady™ is a comprehensive solution designed to revolutionize cardiometabolic disease
clinical trials. Discover how our platform empowers researchers and enhances patient outcomes
through innovation and technology.

GlucoseReady™ will prompt the patient in real time to complete protocol-defined PRO questionnaires following a hypoglycemic event (CGM / BGM). These automatically triggered event questionnaires provide insights into the symptomatology of hypoglycemia as well as its causality.


Leverage the power of CGM and BGM integrated into our platform to gain immediate insights into patient glucose levels. Near real-time data facilitates rapid decision-making and adaptation in trial protocols, aligning with the latest FDA guidance.
Discover more about GlucoseReady™
GlucoseReady™ is not just advanced; it’s precision-engineered to meet new FDA guidance and the exacting standards of today’s cardiometabolic clinical trials.
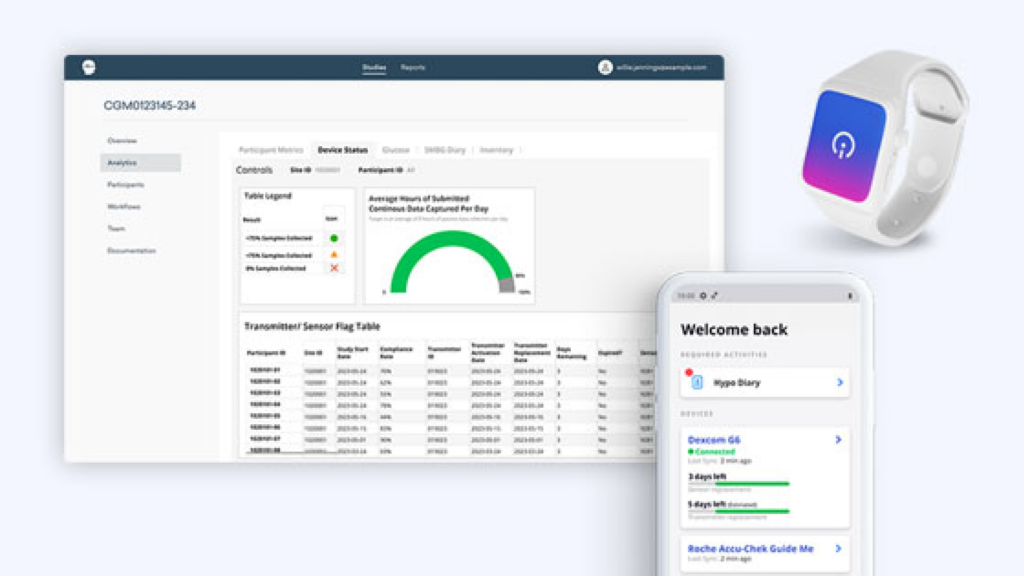
GlucoseReady™ excels in aggregating data from various sources including CGM, BGM, digital weight scales, and actigraphy devices. Our platform synthesizes this data within a cohesive framework, providing comprehensive analytics that support dynamic trial management and informed decision-making.
Designed to meet the rigorous requirements of GCP and FDA guidelines, GlucoseReady™ ensures that all data handling processes uphold the highest standards of data security and patient privacy. Our platform is regularly updated to stay compliant with evolving regulations.
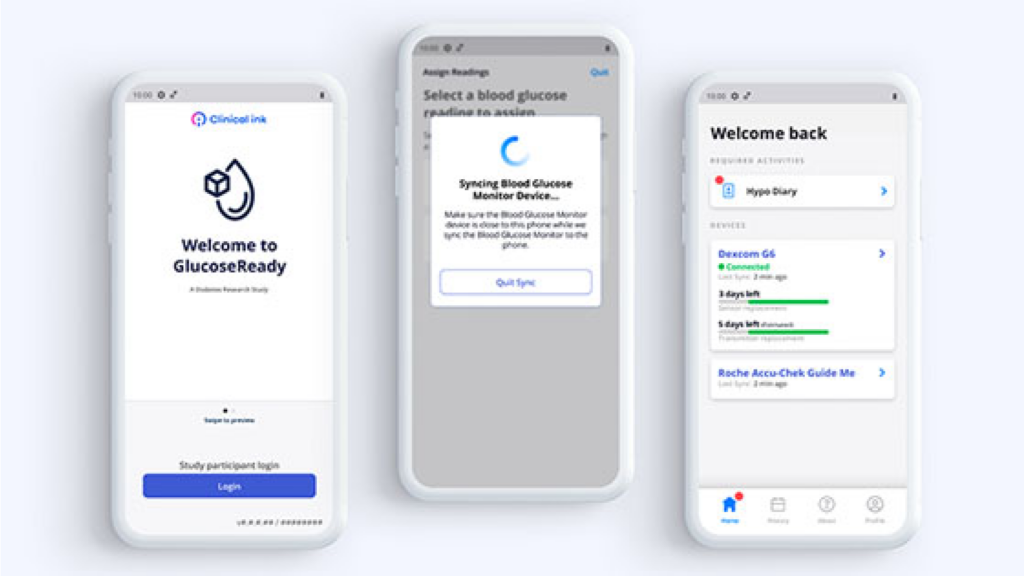
Understanding that the user experience is critical, GlucoseReady™ features an intuitive interface designed for ease of use by both clinical staff and participants. Comprehensive support and training materials are readily available to ensure successful platform adoption.
GlucoseReady™ is packed with innovative features that set new standards in clinical research technology. From predictive analytics that forecast patient compliance to automated triggers for eCOA based on specific data thresholds, our platform is designed to advance the field of cardiometabolic disease management.
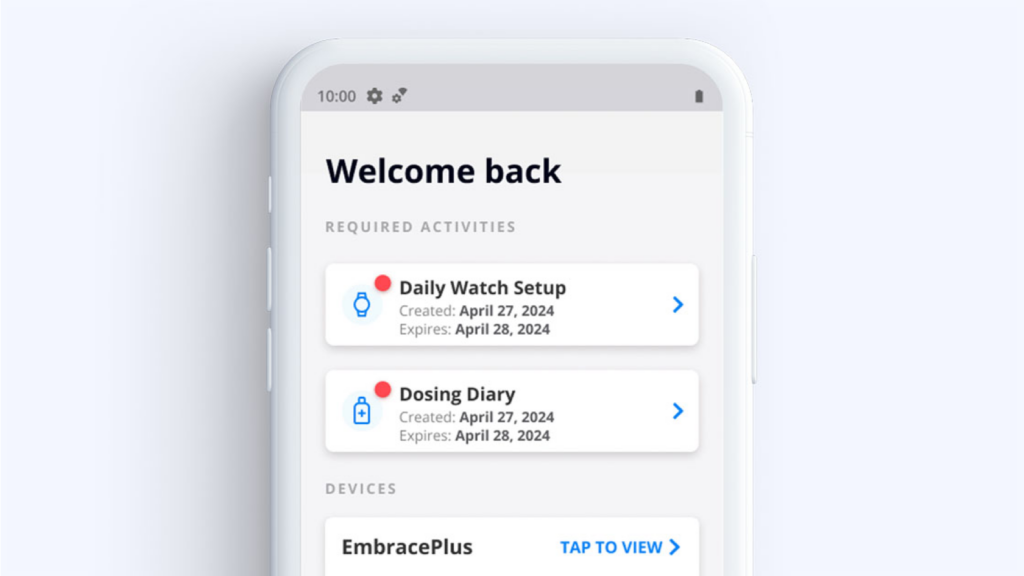
GlucoseReady™ provides modules used separately or together to support multiple therapeutic areas, including within a single GCP platform designed for user-friendliness and the requirements of an FDA inspection.
Download our fact sheet to learn more.
Sales and Solutions
Direct 1.336.464.0697
Support
Toll-Free 1.800.301.5033
Direct 1.336.464.0697
Insert HTML text here.
Download the GlucoseReady™ white paper and see how our platform can streamline data capture, improve retention, and ensure regulatory compliance.
Request more information, submit questions, set up a demonstration, and more.
Introducing GlucoseReady™: Transforming Cardiometabolic Trials