When word processors were introduced, no one expected them to be able to store data and function as our laptops do today. They simply made it easier to type documents—and that was a breakthrough in and of itself. Similarly, when electronic data capture (EDC) systems came onto the clinical trial scene, no one expected them to do anything more than capture the data recorded on paper during patient visits at the research site. That was all they did, and that was enough.
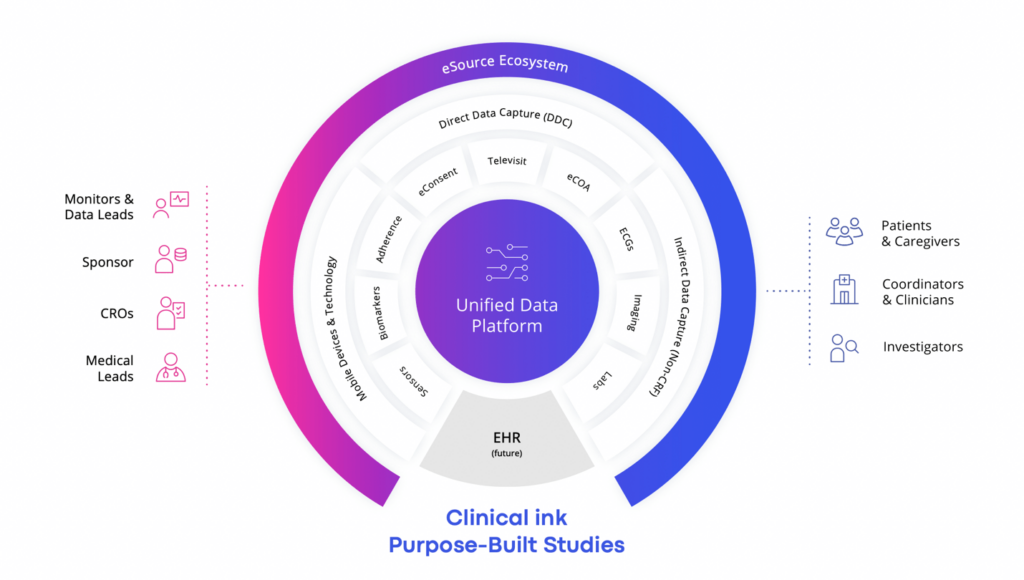
However, how and where trials are conducted is changing, accelerated in part due to the COVID-19 pandemic. Trials have become more decentralized, and data can be collected in many different ways, from televisits to mobile devices, regardless of physical location—patients may not even need to be present at a physical clinical site. Increasingly, source data for clinical trials can include eConsent forms, patient reported outcomes (PRO), clinician reported outcomes (ClinROs), data from eDiaries; eCRFs completed during telehealth, in-home patient visits, or visits to remote medical locations; laboratory reports; imaging; and data from wearables and sensors.
One might assume that the “E” in EDC systems would apply to all of these sources, but it does not. At their core, EDC systems are simply a way for site staff to transcribe data from the patient visit into an electronic record rather than using a paper case report form (CRF). In many cases, a paper source document is still used to record data during the patient’s site visit. This process breaks down when the patient can’t or won’t come to the site.
The one-trick nature of EDCs is inadequate to support many trials, as without heavy, manual integration processes they lack the ability to capture data in real time, and often require patients to visit a research site. Fortunately, technologies exist to ingest electronic data from the various electronic sources, supporting virtual or hybrid studies. A purpose-built eSource solution from Clinical ink supports direct data capture from these many sources into its secure database, shared with the Sponsor, and can be used wherever the patient might be. This provides a comprehensive view of the patient in real time.
Such a system vastly improves the efficiency of capturing, recording, verifying, and sharing data in clinical trials. It reduces bottlenecks for assessments, makes it easy to remotely review source data, supports cleaner data upon entry (with real-time edit checks), and reduces data queries. All of this reduces patient and site burden while reducing both time and cost to the Sponsor, and accelerating database lock.
In one study that Clinical ink supported, for example, the protocol included a combination of on-site and home visits. While the baseline assessment and end-of-study assessments were performed at the site, all other assessments were conducted in the patient’s home via video conferencing. Investigators captured data from those visits, including video recordings, on their tablets and the data were uploaded to the Clinical ink secure, Sponsor-accessible database portal. The Sponsor thus had immediate access to validated data via a portal and there was no lag time between the patient visit and data visibility.
Additionally, it eliminated any reconciliation time over duplicative data sets (as is the case with paper source documents) as well as time for the transcription of source data into an EDC. This in turn reduces queries by Sponsor monitors and site staff, allowing for the real-time monitoring of trial progress and leading to cleaner data all around. Indeed, with Clinical ink eSource Direct Data Capture, the average query rate for entire studies is 0.23%, with an average response time of about one day. All in all, the average time to export data was 28 minutes, and the average database lock time after the last patient’s last visit was only 23 days.
The availability of eSource platforms to capture, integrate, and report on data collected from multiple sources gives Sponsors the ability to truly pursue decentralized study designs or to comprehensively incorporate decentralized elements into their hybrid trials. The utility of eSource platforms should not be confused with the much more limited purpose of some EDC systems where the source of the data remains the paper form completed during the site visit, and which often do not have the ability to capture in-clinic data in real time.
Discover more information on how Clinical ink eSource technology supports all trials—including in-clinic, remote, and hybrid.

Author
Rinah Yamamoto
Principal Scientist, Clinical ink
