People don’t participate in clinical trials in hopes of making more trips to the clinic, changing their daily routines, or struggling with cumbersome or intrusive reporting requirements. At best, these hindrances are “necessary evils” of trial participation. At worst, they’re non-starters for potential participants, the reasons patients stop adhering to the study protocol, or factors that ultimately lead to study discontinuation.
Fortunately, as Sponsors incorporate eSource technologies and decentralized data collection into their trials, these barriers to patient participation and adherence are beginning to fall away. Direct Data Capture (DDC) software can collect patient data at the instant they’re initially recorded in an electronic format—i.e., during telehealth visits, in electronic diaries and patient engagement platforms, and via sensors and wearables.
In the broadest sense, eSource technologies allow Sponsors to center trials on patients and their needs as never before. This advancement of clinical trials can thus be based on comprehensive, actionable, patient-centered measures and assessments, or what Clinical ink calls “patient science.” With the right systems and tools, trials can collect comprehensive and actionable measures that meet research objectives, and therefore advance the pursuit of a treatment or cure. More specifically, with respect to trial participation, software and tools that allow data to be captured electronically give patients:
- Greater flexibility and convenience. When mobile devices are employed, data can be collected from anywhere (even when internet connectivity is temporarily unavailable), meaning that patients need not travel to the clinic for a growing number and type of assessments.
- Increased safety. Wearables and sensors can track and report side effects continuously and automatically for immediate review by safety teams.
- A stronger sense of involvement. Rather than being observed by a clinician during infrequent visits, patients are able to report their own feelings about their condition with greater frequency — an opportunity that they generally appreciate.
Other benefits accrue when eSource tools are designed with patient needs, abilities, and preferences in mind. The best solutions make patient participation easy and engaging by providing:
- Unobtrusive data collection. Many mobile devices, including sensors and wearables, fit seamlessly into patients’ daily lives, and data are collected passively and automatically as patients go about their day. Patients need not take any steps to comply with the data collection requirements.
- Intuitive, approachable applications. When patient reporting tools are developed according to the best practices of user experience (UX) design, assessments are easy for the target patient population to complete. Design elements for electronic symptom diaries, for example, can include radio buttons, emojis, sliding scales, and questionnaires with built-in and transparent skip logic. Active assessments utilizing sensors and wearables, for instance on an Apple Watch, similarly engage patients with simple, guided, and easy-to-complete mobile activities. The best approaches are not coldly clinical in nature, but pleasant — and at times even fun — to complete.
- Simplified assessments. Patients (as well as clinicians) can more easily navigate complex protocols when measurements are focused on those that will get the best (most meaningful) signal, such as, for example, the BRACE (BrainBaseline™ Assessment of Cognition & Everyday Functioning) used to test patients’ cognitive and mental health. Dr. Rubin, an Associate Professor of Neurology, Psychiatry, and Epidemiology, says, “Being able to hand an iPad over and have [patients] complete the BRACE cognitive battery basically [takes] away all of the burden.”
- Easy instructions. We recommend creating video instructions for patients to help them complete assessments either at home or in the clinic. Clear, animated video clips are helpful, for instance, in demonstrating the steps a patient must take to complete cognitive, small motor, mobility, verbal articulation, and sustained phonation tests. Such instructional videos give patients confidence that they’re proceeding correctly, and ultimately improve the quality of the data.
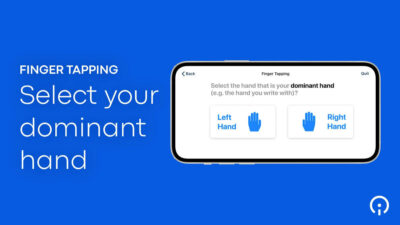

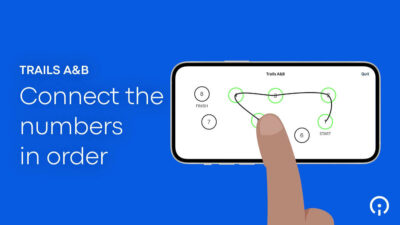
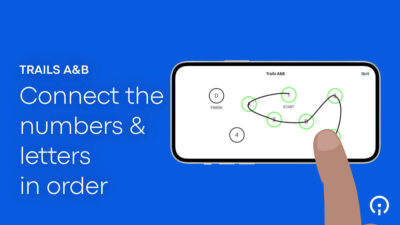
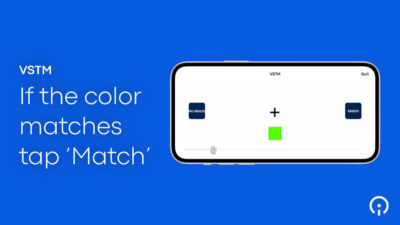
All of these innovations allow Sponsors to meet patients “where they’re at” — physically, cognitively, psychologically, and practically speaking. They enable patients, by leveraging technology as well as their own expertise, to fully participate in patient science. Patients are required to make fewer visits to the clinic and can provide their data either through completely passive means, or through active measures that are both easy and engaging. When eSource tools are properly designed and integrated into a trial’s protocol, they offer patients a seamless trial experience that is attentive to their needs, accommodates their limitations, and reinforces their commitment to the research.
